Highlights:-
•Parkinson’s Foundation report that about 1 million people had the disease in 2020, with about 10 million affected people over the world. Despite this prevalence, scientists are still unsure why Parkinson’s disease affects some people and not others, and there is currently no cure.
•This type of brain disorder typically affects people over the age of 60, and the symptoms worsen with time. Common symptoms include stiffness, difficulty walking, tremors, and trouble with balance and coordination. The disease can also affect the ability to speak and lead to mood changes, tiredness, and memory loss.
•Experiments of some researchers found that the bone morphogenetic proteins 5 and 7 (BMP5/7) can have these effects in a mouse model of the disease. This research may be the first step toward developing a new treatment for Parkinson’s disease.
What is Parkinson’s disease?
A movement disorder of the nervous system that worsens over time. As nerve cells (neurons) in parts of the brain weaken or are damaged or die, people may begin to notice problems with movement, tremors, stiffness in the limbs or the trunk of the body, or impaired balance. As these symptoms become more obvious, people may have difficulty walking, talking, or completing other simple tasks. Not everyone with one or more of these symptoms has PD, as the symptoms appear in other diseases as well.
No cure for PD exists today, but research is ongoing and medications or surgery can often provide substantial improvement with motor symptoms.
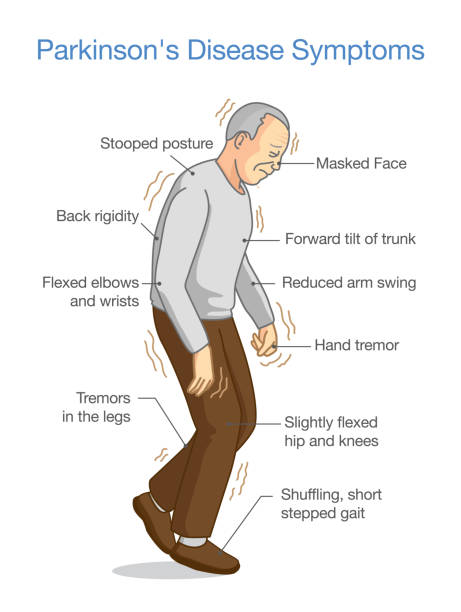
Symptoms of the disease:-
The four primary symptoms are:-
•Tremor- Shaking often begins in a hand, although sometimes a foot or the jaw is affected first. The tremor associated with PD has a characteristic rhythmic back-and-forth motion that may involve the thumb and forefinger and appear as a “pill-rolling.” It is most obvious when the hand is at rest or when a person is under stress.
•Rigidity- Muscle stiffness, or resistance to movement, affects most people with PD. The muscles remain constantly tense and contracted so that the person aches or feels stiff.
•Bradykinesia - This slowing down of spontaneous and automatic movement is particularly frustrating because it may make simple tasks difficult. The person cannot rapidly perform routine movements. Activities once performed quickly and easily—such as washing or dressing—may take much longer.
•Postural instability- Impaired balance and changes in posture can increase the risk of falls.
Who gets Parkinson’s disease? & what causes the disease?
The average age of onset is about 70 years, and the incidence rises significantly with advancing age. However, a small per cent of people with PD have “early-onset” disease that begins before the age of 50. People with one or more close relatives who have PD have an increased risk of developing the disease themselves. An estimated 15 to 25 per cent of people with PD have a known relative with the disease. Some cases of the disease can be traced to specific genetic mutations.
The cause of PD is unknown, although some cases of PD are hereditary and can be traced to specific genetic mutations. Most cases are sporadic—that is, the disease does not typically run in families. It is thought that PD likely results from a combination of genetics and exposure to one or more unknown environmental factors that trigger the disease. Although many brain areas are affected, the most common symptoms result from the loss of neurons in an area near the base of the brain called the substantia ‘nigra’. Normally, the neurons in this area produce dopamine. Dopamine is the chemical messenger responsible for transmitting signals between the substantia ‘nigra’ and the next “relay station” of the brain, the corpus striatum, to produce smooth, purposeful movement. Loss of dopamine results in abnormal nerve firing patterns within the brain that cause impaired movement.
People with Parkinson’s have lost 60 to 80 per cent or more of the dopamine-producing cells in the substantia nigra by the time symptoms appear. People with PD also lose the nerve endings that produce the neurotransmitter norepinephrine- the main chemical messenger to the part of the nervous system that controls many automatic functions of the body, such as pulse and blood pressure. The loss of norepinephrine might explain several of the non-motor features seen in PD, including fatigue and abnormalities of blood pressure regulation.
How is Parkinson’s disease diagnosed?
Parkinson’s disease is a slowly progressive disorder. It is not possible to predict what course the disease will take for a person. The average life expectancy of a person with PD is generally the same as for people who do not have the disease. Fortunately, there are many treatment options available for people with PD. However, in the late stages, PD may no longer respond to medications and can become associated with serious complications such as choking, pneumonia, and falls.
There are currently no specific tests that diagnose PD. The diagnosis is based on:
•Medical history and a neurological examination
•Blood and laboratory tests, to rule out other disorders that may be causing the symptoms
•Brain scans to rule out other disorders. However, computed tomography (CT) and magnetic resonance imaging (MRI) brain scans of people with PD usually appear normal.
In rare cases, where people have an inherited form of PD, researchers can test for known gene mutations as a way of determining an individual’s risk of developing the disease. However, this genetic testing can have far-reaching implications and people should carefully consider whether they want to know the results of such tests.
Treatment & Surgery
Medications for Parkinson’s include:
Levodopa or Carbidopa, the cornerstone of therapy for PD is the drug levodopa (also called L-dopa). Nerve cells can use levodopa to make dopamine and replenish the brain’s reduced supply. People cannot simply take dopamine pills because dopamine does not easily pass through the blood-brain barrier. (The blood-brain barrier is a protective lining of cells inside blood vessels that regulate the transport of oxygen, glucose, and other substances in the brain.)
Usually, people are given levodopa combined with another substance called carbidopa. When added to levodopa, carbidopa prevents the conversion of levodopa into dopamine except for in the brain; this stops or diminishes the side effects due to dopamine in the bloodstream. Levodopa or carbidopa is often very successful at reducing or eliminating the tremors and other motor symptoms of PD during the early stages of the disease.
The earliest types of surgery for PD involved selectively destroying specific parts of the brain that contribute to PD symptoms. Surgical techniques have been refined and can be very effective for the motor symptoms of PD. The most common lesion surgery is called pallidotomy. In this procedure, a surgeon selectively destroys a portion of the brain called the globus pallidus. Pallidotomy can improve symptoms of tremor, rigidity, and bradykinesia, possibly by interrupting the connections between the globus pallidus and the striatum or thalamus. Some studies have also found that pallidotomy can improve gait and balance and reduce the number of levodopa people require, thus reducing drug-induced dyskinesias. Another procedure, called thalamotomy, involves surgically destroying a part of the thalamus; this approach is useful primarily to reduce tremors.
Deep brain stimulation, or DBS, uses an electrode surgically implanted into part of the brain, typically the subthalamic nucleus or the Globus pallidus. DBS does not stop PD from progressing, and some problems may gradually return. While the motor function benefits of DBS can be substantial, it usually does not help with speech problems, “freezing,” posture, balance, anxiety, depression, or dementia. DBS is generally appropriate for people with levodopa-responsive PD who have developed dyskinesias or other disabling “off” symptoms despite drug therapy.
What research is being done? To ‘slow or stop’ disease progression The mission of the National Institute of Neurological Disorders and Stroke (NINDS) is to seek fundamental knowledge about the brain and nervous system and to use the knowledge to reduce the burden of neurological disease. NINDS is a component of the National Institutes of Health (NIH), the leading supporter of biomedical research in the world. NINDS conducts and supports three types of research: basic scientific discoveries in the lab, clinical developing and studying therapeutic approaches to Parkinson’s disease, and translational focused on tools and resources that speed the development of therapeutics into practice. The goals of NINDS research on Parkinson’s disease are to better understand and diagnose PD, develop new treatments, and ultimately, prevent PD.







